Last Updated on November 23, 2025 by Brian Beck
🌱 **The Hidden Chemistry of Your Lawn:
Why Nutrients Get Stuck, Locked Out, or Work Against Each Other**
Most people think lawn care is about “adding fertilizer.”
But the real story—the one nobody in the synthetic world ever explains—is that your lawn is fed by a complex nutrient ecosystem, and every single element interacts with the others. Get one of them out of balance and the whole system misfires.
This is why some lawns green up easily while others fight you every step of the way, even when you’re “doing everything right.”
Today we’re pulling the curtain back on the full nutrient system of turfgrass, what each element does, and—more importantly—what happens when things get excessive or get locked out by pH, salt, compaction, or other antagonistic elements.
Think of this as the ultimate nutrient truth guide for homeowners who want to understand why their soil matters and why traditional fertilizing often fails.
🌿 The Elements Your Grass Actually Uses
Grass doesn’t need “a fertilizer.”
It needs 17 essential elements—some in large quantities, others in microscopic doses. These fall into three groups:
Macronutrients (needed in large amounts)
-
Nitrogen
-
Phosphorus
-
Potassium
-
Calcium
-
Magnesium
-
Sulfur
These build structure, color, roots, and resistance.
Micronutrients (needed in tiny amounts)
-
Iron
-
Manganese
-
Zinc
-
Copper
-
Boron
-
Molybdenum
-
Chlorine
-
Nickel
These act like spark plugs. Small amounts make or break turf health.
Beneficial nutrients
-
Silicon
-
Cobalt
-
Sodium (in trace, trace amounts)
Not essential, but they help biology, stress tolerance, and structural strength.
Sounds simple, right?
It’s not.
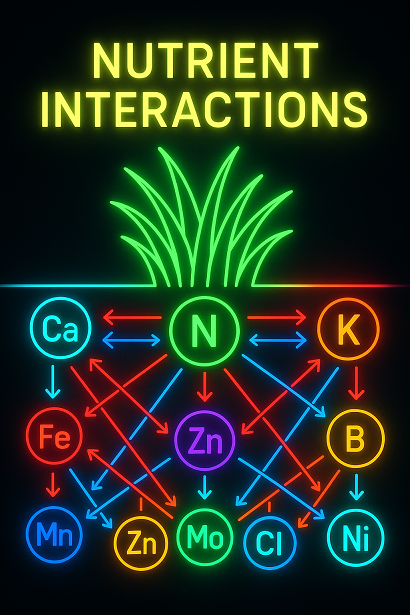
Because every single one of these elements can become a problem when they’re out of sync.
⚠️ When “Enough” Turns Into “Too Much”: Excess Nutrients & Turf Damage
Here’s the part synthetic fertilizer companies never talk about:
Excess nutrients cause lockouts.
Meaning: you can have nutrients in the soil—lots of them—and your lawn still starves because something else is blocking their uptake.
This is the “silent killer” of turf performance.
✔ Too much nitrogen?
Shallow roots, water addiction, disease pressure, locked-out potassium and iron, weak structure.
✔ Too much phosphorus?
The big one. It locks out zinc, iron, manganese, and copper. Turf turns yellow no matter how much you feed it.
✔ Too much potassium?
Blocks magnesium and calcium. Turf becomes brittle, weak, drought-sensitive.
✔ Too much calcium?
Raises pH and locks out almost every micronutrient. Soil becomes tight, hard, droughty.
✔ Too much magnesium?
Compacts the soil and locks out calcium and potassium.
✔ Too much iron or manganese?
Can shut down each other and suppress phosphorus.
Every nutrient has an “enemy.”
And almost all of these excesses come from one thing: the synthetic approach.
🔒 The Great Lockout Problem: Why High pH Makes Lawns Look Sick
High pH—very common in Colorado—is the single biggest reason lawns:
-
turn yellow
-
struggle with heat
-
won’t green up
-
use too much water
-
“never seem to respond” to fertilizer
Once pH drifts into the 7.8–8.5 range, the following nutrients basically disappear from the plant’s perspective:
-
Iron
-
Manganese
-
Zinc
-
Copper
-
Boron
-
Phosphorus
-
Silicon
They’re in the soil…
you can measure them on a lab test…
but the grass can’t use them.
This is why a synthetic program can never truly fix a lawn in high-pH soil.
It’s like throwing groceries at a locked refrigerator.
🧪 How Nutrient Lockouts Affect Real-World Lawn Performance
When nutrients are out of balance, the turf reacts in very predictable ways:
Shallow roots
→ Caused by excess nitrogen, phosphorus, or compaction.
Increased water use
→ Deficient potassium or locked-out calcium makes turf thirsty.
Yellowing that won’t go away
→ High pH + phosphorus excess shuts down iron, zinc, and manganese.
Disease susceptibility
→ High nitrogen + low calcium + low silica equals fragile, weak turf.
High costs
→ Turf becomes dependent on fertilizers, fungicides, and water.
This is why “more product” never solves the underlying problem.
Until the soil chemistry and biology are aligned, you’re always bailing water out of a sinking boat.
🌱 The Trinity Difference: Balance Instead of Band-Aids
The biological route fixes nutrient problems the only way they can be fixed:
-
Rebalancing antagonistic nutrients
-
Reducing the excesses that cause lockouts
-
Lowering pH naturally through microbial activity
-
Increasing humus so nutrients actually stay plant-available
-
Improving water efficiency by strengthening the soil structure
-
Using biology to unlock what synthetic fertilizers can’t
Instead of forcing growth, we restore the nutrient engine so the lawn feeds itself—just like nature intended.
This is why Trinity lawns use:
-
30–50% less water
-
Far fewer inputs
-
Less mowing stress
-
Less disease pressure
-
More resilience
-
Better color with less product
You’re not feeding a lawn.
You’re waking up a soil system.
🌎 Final Thought: Your Lawn Isn’t Deficient—It’s Blocked
Most lawns don’t suffer because they don’t have nutrients.
They suffer because nutrients are:
-
tied up
-
antagonized
-
locked out
-
imbalanced
-
blocked by pH
-
drowned by salts
-
suppressed by compaction
Your soil test doesn’t tell a story of “more fertilizer needed.”
It tells a story of unlocking what’s already there.
And that’s exactly what the Trinity system is built to do.
Read more here: